No extra sample prep – just gentle redispersion
Reliable Particle Standards for Accurate Calibration and Quality Control
Opti-Count® is a ready-to-use particle number concentration standard with 400 nm polystyrene particles in a surfactant-free, water-based buffer. It is available at concentrations of 10⁶ or 10⁸ particles/ml and supplied in a 1 mL single-use vial. Only gentle redispersion is needed before use—no further preparation required.
With an expanded combined uncertainty of less than 15%, determined using ISO 17025-accredited methods. Opti-Count® is ideal for method validation, device calibration, and quality control. It supports applications in pharmaceuticals, cosmetics, pigments, inks, emulsions, and other nanotechnology-based products.
Highlights
RELIABLE, TRACEABLE AND EASY TO USE
- Certified particle number concentration -
nominal 106 and 108 particles/ml - Expanded combined uncertainty*: <15 %
- Monomodal nominal 400 nm polystyrene particles - traceable to SI units.
- Surfactant-Free: Electrostatic stabilization in an aqueous medium
means low background noise - Ready-to-Use
- Storage at room temperature
*A combination of the uncertainties from homogeneity, short-term stability and long-term reproducibility (k=2); determined using accredited methods.

More information
We are pleased to provide advice on Opti-Count®.
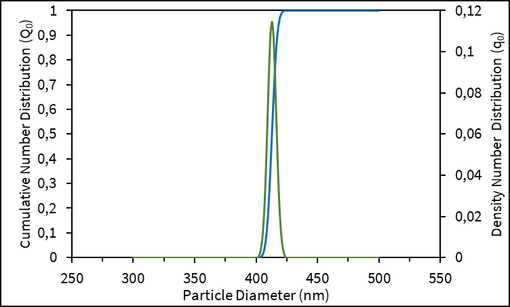
Opti-Count®: Precision in Particle Size Distribution
Opti-Count® particles provide indicative values for the number-weighted size distribution (PSD), offering a more detailed picture of your sample’s composition. Number-weighted distributions highlight the true abundance of smaller particles that may otherwise be normally overlooked — giving you critical insights into product quality and performance.
Analysis of Opti-Count® particles were obtained using the LUMiSpoc® single-particle liquid laser counter, an advanced instrument based on cutting-edge cytometry technology. This ensures reliable, high-resolution detection of particles across a wide size range.

Whitepaper
Use of Reference Materials for Nanomaterial Regulation
As the use of nanomaterials grows across industries, so do concerns about their impact on human health and the environment, especially in the case of nanoplastics. Yet despite increasing regulatory efforts, there remains a critical gap: no standardized reference materials exist for measuring nanoscale (<1 micrometer) particle number concentrations and number-weighted size distributions. This white paper explores the current challenges in defining, detecting, and regulating nanomaterials, and introduces Opti-Count® particle reference materials – a new particle-based solution designed to fill to support consistent, trustworthy, and comparable particle characterization at the nanoscale.
More information
We are happy to advise you on Opti-Count®.
Downloads & Technical facts
Technical facts Opti-Count®
| Applications | Validation, Calibration, QC, Monitoring |
| Concentration | Nominal 106 and 108 particles/mL available |
| Particle Size | Nominal 400 nm |
| Materials | Spherical, polystyrene particles that are electrostatically stabilized in an aqueous buffered solution |
| Surfactant-Free | yes |
| Stability | 9 months after production date guaranteed |
| Expanded Uncertainty | <15% (concentration), <8% (size) |
| pH Value | 8 |
| Density | 1.05 g/cm³ |
| Refractive Index | 1.62 @ 405 nm, 1.59 @ 589 nm; 25°C |
| Packaging | Single-use plastic vials (1 mL) |
| Package Units | 1 x 1 mL and 6 x 1mL available |
| Storage | Store at upright at room temperature and keep vials closed tightly |
| Ready-to-Use | Gentle redispersion before use is sufficient |
Sample Certificate
Reference Material
Each reference material is accompanied by a Certificate of Analysis (CoA) that provides detailed information about the material’s properties. This includes the certified number concentration, indicative quantiles of the number-weighted particle size distribution (x₁₆,₀ x₅₀,₀ x₈₄,₀), and a complete measurement uncertainty budget. All values are traceable to SI units. The certificate also includes additional material characteristics such as density, refractive index, transmission electron microscopy (TEM) analysis, and guidelines for proper storage and use. The CoA ensures the accuracy, reliability, and traceability of the reference material, making it a critical resource for method validation, instrument calibration, quality control, and regulatory compliance in a wide range of analytical applications.
